Respected and reliable longitudinal anonymised and generalised patient data

THIN® is a respected and reliable data source in the fields of research and academic studies, providing anonymised patient data for use in prospective studies, disease specific case studies and retrospective studies.
THIN® has been cited in over 1,700 publications and that list continues to grow with around 20 new reports added every month.

THIN Data Uses
Academics, scientists and pharmaceutical companies seeking access to primary care medical record data have used THIN for many reasons including but not limited to;
- Accelerate drug discovery and development
- Improve the effectiveness of clinical trials
- Target a specific population of patients
- Utilise the behavioral insights in relation to drug effectiveness and healthcare outcomes
- Improve safety and risk management
- Provide insight into marketing and sales performance (Pharma)
Academic Institutions
THIN works closely with the University of Birmingham and University College London, both of whom form part of our governance committees.
Institutions and departments within universities can access data from THIN for a variety of research areas, including having full access to the THIN database or a select data extract through our parent company and partner Cegedim Health Data

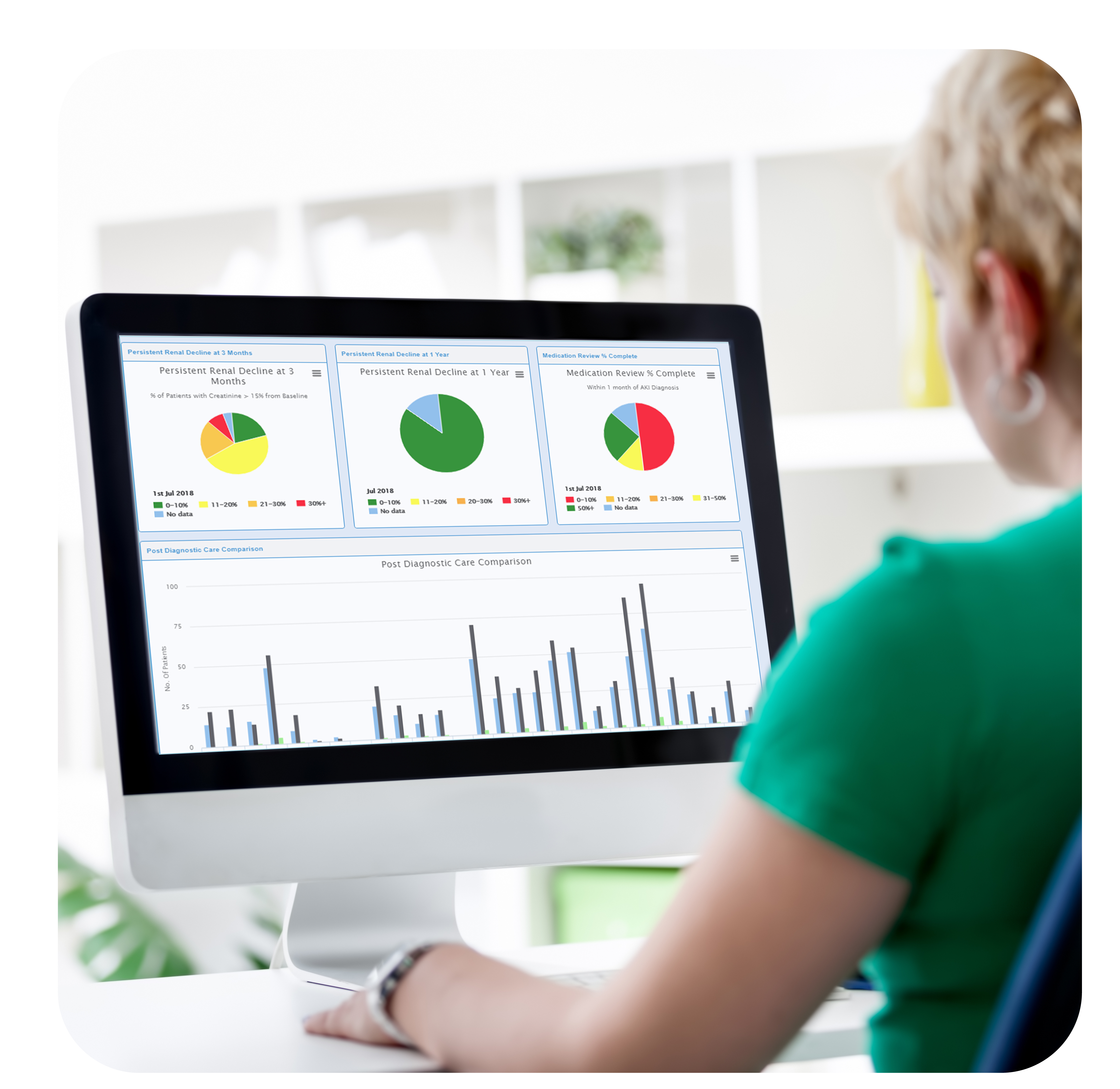
Unique areas in which Academia can have access to include:
- THIN Research Services: GP Questionnaires, Patient Questionnaires, Redacted notes from Electronic Health Records, Death Certificate analyses.
- Affecting Change through Cegedim’s Outcomes Manager solution which is system agnostic. This allows critical research to be put into practice where change can be affected at the patient or clinician level.
- Clinical Trial scoping, development and implementation – utilising data from THIN in addition to Cegedim’s Outcomes Manager solution.
Data Governance
The THIN® Advisory Committee provides advice on the operations of the database, to ensure that it continues to be used in a way that maximises its research value in the best interests of the public whilst protecting the rights of its data subjects.
Our Advisory Committee meets three times each year, or as needed, and consists of representatives from the following organisations and roles:
• THIN® management employed by Cegedim
• Data scientists and epidemiologists from THIN® academic partners
• NHS clinicians, including general practitioners
• Patient representatives
In addition, we work to the highest of regulatory standards.
The regulatory bodies we have approval from include:
FAQ's
- What information does THIN® collect?
Registration Details, patient encrypted ID, year of birth, sex, registration dates. For patients less than 1 year old, the month as well as year of birth is collected. Therapy records, acute and repeat prescriptions, dates and issues, including prescribing instructions, drug codes and dosages. Medical history records and clinical codes. Additional health data, including blood tests and other examination results.
- Real World Evidence - how is THIN data used for research?
The collected Real-world Data is used mainly to carry out studies including scientific and statistical studies, producing Real-world Evidence to inform research. This can either be directly by THIN® or by a third-party entity to whom the data is transmitted in anonymised form. These studies are mainly intended for the Life Sciences sector, the medical and academic world as well as research centres. THIN® has been cited in over 1,700 publications and that list continues to grow with around 20 new reports added every month.
Contact our research data team
Complete the form below or you can email us directly CHD-Operations@cegedim.co.uk



